Our Research and Development Department is part of the Medical Directorate. We are based in the Clinical Translational Research & Innovation Centre (C-TRIC), which is located on the Altnagelvin Hospital site. Our goal is to support and promote high quality clinical research for the health benefits of our population and to play our part in the delivery of research studies, and to align with the regional research and development (R&D) strategy for the Health and Social Care (HSC) sector.
The role of the department is to support and facilitate researchers to undertake high quality research and to provide research governance to ensure the interests of our participants, researchers and staff adhere to the UK Policy Framework for Health and Social Care Research.
We work closely with researchers from the Ulster University and Queen’s University as well as developing collaborative working with our three research network partners,
Northern Ireland Clinical Research Network (NICRN)
Northern Ireland Cancer Trials Network (NICTN)
Northern Ireland Public Health Research Network (NIPHRN)
-
Our Service
Our service is directed mainly at external researchers/applicants/sponsors of health research studies; to our staff who wish to conduct studies related to their own specialty/disease area of interest; as well as to the public who may wish to get involved in health research related activity, locally or through the regional group of representatives that consider public involvement enhancing research (PIER).
-
Further Resources
A list of current and open studies can be found by contacting Catriona Lavery, Research Nurse Manager.
We are also recruiting on behalf of Genomics Medicine Ireland to a range of studies across a number of disease topics – view opportunities.
We encourage public participation in research, and this means involving people in all aspects of the research process as partners rather than as research subjects – more information on public participation in research.
We work closely with the Research Division of the Public Health Agency and with the Health Research Authority
-
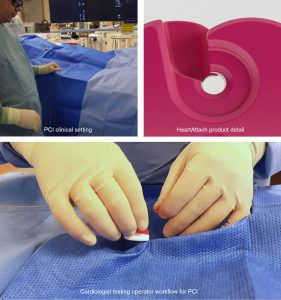
HeartAttach: Cardiology Guidewire Separator
The Cardiology department at the Western Trust has teamed up with Ulster University’s Belfast School of Art to co-design a new device to improve the clinical operator workflows for Percutaneous Coronary Intervention (PCI). Dr Aaron Peace, (cardiologist) and Dr Justin Magee (product designer) have worked together towards innovative new product development and subsequently, workflow service improvements.
PCI is a procedure where stents are inserted into narrowed heart blood vessels that limit blood flow. A tactile responsive coronary guidewire, usually measuring 0.014’ in diameter is used to guide stents into final position which results in widening and increased blood flow – resulting in relief of symptoms for patients. In Europe there are 1.1 Million PCI’s (2018) with 27.7K increase per year (Eurostats, 2020). A major workflow issue arises in that the wires, regardless of manufacturer, look very similar in appearance. They are difficult to see and it can be difficult to recall which wire is in which blood vessel especially in dimly lit catheterisation laboratories or operation theatres. This adds to the cognitive clutter that an operator experiences while viewing moving real time images of the heart on screen, while simultaneously performing an procedure below their line of vision. The associated uncertainty adds to the increased risk of medical errors which are a major concern throughout the clinical profession. Within this context ‘cardiac catheterization are significant sources of medical cardiovascular liability’ (Rodziewicz and Hipskind 2020). Currently interventional cardiologists may use a piece of gauze to help identify a specific guidewire as no current solution is adequate to enable an uninterrupted operator workflow. Furthermore, a gauze may accidentally be removed during an operation during hygiene protocols, incurring corrective delays and error may introduce microfilaments into the body.
The Design and its Benefits
Observation analysis of the operator procedures was conducted at Western Trust. This informed design decision making within a co-creative process between design and clinical experts. An innovative product was iteratively developed under critical review and functional testing by a team of consultant cardiologists. Novel features were defined and incorporated permitting normal operative workflow of individual guidewires. The benefits of the novel product design includes:
- Enablement of an unobtrusive workflow environment permitting normal operative procedures
- Visually identifies the artery of insertion, clarifying which wire is in which artery.
- Permits one hand relocation and full control of the guidewire while the device is in use
- Avoids entanglements and unintentional removal errors related to guide wires where multiple wires are in use.
- Avoids touching or over manipulation of the wire thus avoiding kinking and damage to the wire
- Offers guidewire management where multiple wires are in use for all diameters of wires and associated equipment.
- Any vascular procedure using any wire diameter can benefit from this design system.
The HeartAttach product addresses this clinical need. The improvements embodied in this product meet recommendation 240 of the Francis Inquiry (Francis, 2013) including improvement of clinical workflow management, operative communication, hygiene best practice, and reduction of cognitive clutter.
The product has been developed through several prototype variations following clinical feedback, and exists as an injection moulded prototype.
Support
The research has been funded by Health & Social Care for Northern Ireland (HSCNI), Western Trust Research and Development Fund and Ulster University including the Higher Education Innovation Fund (HEIF) Commercial Impact Award. HSC Innovations facilitated discussions with potential commercial partners with confidentiality agreements in place.
References
Francis R (2013) Report of the Mid Staffordshire NHS Foundation Trust Public Inquiry: Executive summary
Rodziewicz TL, Hipskind JE. (2020), Medical Error Prevention. In: StatPearls. Treasure Island (FL): StatPearls Publishing
-
Contact Us
Research & Development Department
C-TRIC
Altnagelvin Hospital
Glenshane Road
Londonderry BT47 6SB
028 7161 1362
Email: research.office@westerntrust.hscni.netLead Contact: Catriona Lavery, Research Nurse Manager
Caitriona.Lavery@westerntrust.hscni.net




